Understanding the evolution of Barrett’s oesophagus to cancer
February is Oesophageal Cancer Awareness Month. Oesophageal cancer develops in the tube that links the mouth to the stomach (oesophagus), and is diagnosed in approximately 9,300 people in the UK each year1. At Barts Cancer Institute, Queen Mary University of London, a key area of research focus is on a condition that can precede oesophageal cancer called Barrett’s oesophagus.
The precise prevalence of Barrett’s oesophagus or ‘Barrett’s’ is not known, but experts predict that in the UK less than 1% of people have the condition2. Of those with Barrett’s, 3-13% will develop a type of oesophageal cancer, called oesophageal adenocarcinoma, in their lifetime3. Barrett’s is the only known precursor of oesophageal adenocarcinoma and, although the annual risk of cancer development is low, every patient is enrolled in invasive and expensive endoscopic surveillance. Unfortunately is it currently not possible to predict which patients with Barrett’s will go on to develop cancer and this is because we do not fully understand how Barrett’s evolves into cancer.
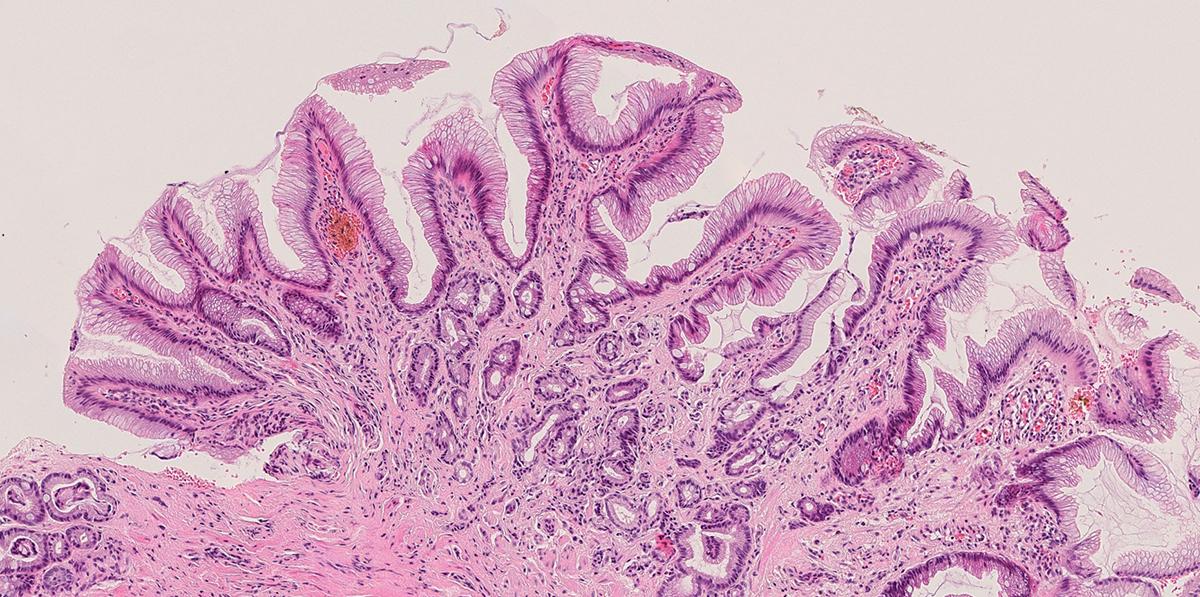
BCI’s Dr Stuart McDonald and his team are working to understand the processes involved in the evolution of Barrett’s to cancer. Findings from the team’s recent study, published in Gastroenterology, suggest that measuring the diversity of the cellular structures (or phenotypes) found in Barrett’s may provide the means to determine cancer risk before progression occurs.
Barrett’s develops when the normal cells that line the oesophagus (known as squamous cells) are replaced by columnar-shaped cells that form ‘glandular’ tissue structures, resembling those found in the intestine and stomach. This is thought to be an adaptive response to chronic bile and acid reflux. The progression to cancer is a stepwise process that involves Barrett’s undergoing a pre-cancerous change called dysplasia, which may progress into oesophageal adenocarcinoma.
The appearance of Barrett’s under the microscope can vary between and within individuals. In the study - funded by the charities Guts UK and Cancer Research UK – Dr McDonald’s team set out to measure the extent of diversity in Barrett’s, to investigate if transitions from one phenotype to another represent an evolutionary process and to determine whether this diversity is associated with progression to cancer.
By examining 64 biopsy samples collected from 51 patients with Barrett’s with no dysplasia or cancer, the team identified five distinct types of glandular structure within the tissue. Lineage tracing experiments using high-resolution laser microdissection combined with the sequencing of the energy-producing structures (mitochondria) within captured cells surprisingly revealed that the Barrett’s glandular structures were able to evolve from one type to another. The team believes this evolution is in response to changes in the environment surrounding the cells.
The team then compared a further 167 biopsy samples taken from three groups of patients: Patients with no dysplasia or history of dysplasia, patients with high-grade dysplasia, and patients who eventually developed dysplasia. The analyses revealed that in patients that go on to develop dysplasia, the diversity of the glandular structures is increased, even prior to progression to dysplasia.
Tools for predicting cancer risk
Dr McDonald said:
“Our data show that the Barrett’s oesophagus tissue structures represent an evolutionary process and suggest that diversity of gland appearance may play a role in progression.”
Endoscopic surveillance monitors a patient’s condition and aims to detect the emergence of dysplasia or cancer at an early stage; however, the majority of patients never progress to cancer and there are no tools for predicting cancer risk.
The findings from the study suggest that tissue diversity may indicate risk of progression, and that understanding how diversity affects patients who progress to dysplasia may prove an important predictive biomarker of cancer risk.
Dr McDonald added:
“Many pre-cancerous diseases show diverse appearances within and between patients. Investigating the relationship of cellular diversity may provide stratification tools for other conditions where the risk of cancer is difficult to predict.”
The team is now investigating the relationship between the Barrett’s phenotype and genetic makeup prior to the development of dysplasia and cancer, and hopes soon to be in a position to understand the complex relationship between the two in order to provide biomarkers that can predict cancer risk.
References
1Cancer Research UK - https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer#heading-Zero Accessed Feb 2022
2Macmillan Cancer Support - macmillan.org.uk/cancer-information-and-support/worried-about-cancer/pre-cancerous-and-genetic-conditions/barretts-oesophagus Accessed Feb 2022
3Cancer Research UK - https://www.cancerresearchuk.org/about-cancer/other-conditions/barretts-oesophagus/about-barrett%27s Accessed Feb 2022
More information
- Research paper: Clonal Transitions and Phenotypic Evolution in Barrett’s Esophagus. Gastroenterology (2021) Dec 29;S0016-5085(21)04167-6. doi: 10.1053/j.gastro.2021.12.271.
- Find out more about the oesophageal cancer research at Barts Cancer Institute
Category: General News, Publications

No comments yet