New insight into how a faulty protein drives lung cancer gives clues to why some therapies fail
A faulty version of a molecule called MET (known as METex14) can drive the development of cancer without the need for any other genetic mutations, according to new research. The scientists also found that this faulty MET needs to be switched on by another molecule called HGF to drive cancer development. These findings, published in Molecular Oncology, could help explain why cancer drugs that target MET work for some people but not others and could inform how doctors decide which treatments are most suitable for different patients.
The study was co-led by researchers at Barts Cancer Institute at Queen Mary University of London, and at the CANTHER unit from ONCOLille Institute and the Centre Hospitalier Universitaire de Lille, France.
MET is a protein that plays an important role in healthy growth, development and wound healing. However, faults in MET can stimulate the growth, survival and spread of many types of cancer, making it an important target for treatment. Several cancer therapies that inhibit MET have already entered the clinic, and companies around the world are developing and trialling many more varieties of these promising drugs. However, MET inhibitors only work in certain people, and it’s unclear how we can predict who will benefit and who will not. We need to better understand how MET functions in cancer to inform how to personalise treatment – selecting the drugs with the best chance of working for each individual.
“When a clinician assesses a person with cancer, they need to ask themselves: what mutation is likely driving the disease, and what do I have on my shelf of personalised therapies that I can use to treat that person?” explains Dr Stephanie Kermorgant, co-senior author of the new study along with Dr Zoulika Kherrouche.

METex14 – removing the brake pedal for cancer development
The team focussed on METex14, a faulty form of MET recently discovered to play a role in some lung, stomach, bowel and brain cancers. METex14 lacks an entire section of the healthy protein that would ordinarily help to switch it off. Without this brake pedal, METex14 becomes over-active, uncontrollably stimulating certain processes within cells.
“Whether METex14 can drive cancer on its own has remained elusive,” say Dr Kermorgant and Dr Kherrouche. “So we decided to create a pure model that isolates METex14 from other gene mutations that might affect tumour development.”
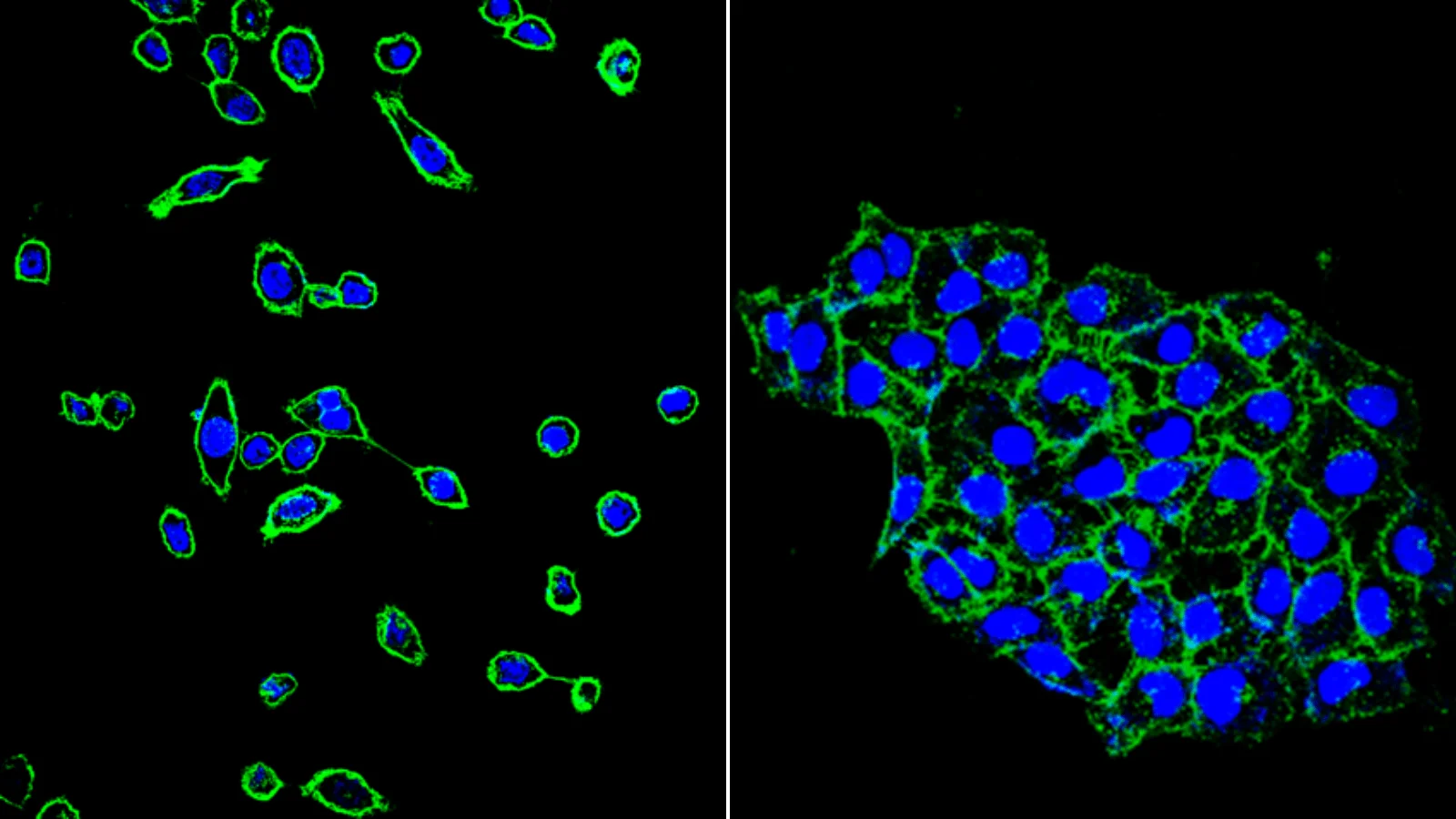
Using a state-of-the-art genetic engineering tool called CRISPR, the researchers edited the DNA of human lung cells, inserting METex14 but no other cancer-driving mutations. This is in contrast to previous studies by other groups, which examined cells that contained METex14 in addition to a number of other mutations, making it difficult to isolate the effect of METex14 alone.
In their pure model, the team saw that METex14 alone could make cells begin to migrate and invade the tissue around them, recreating the behaviour of cancer cells when they spread. But METex14 could only produce this effect when another molecule, called hepatocyte growth factor (HGF), was present. HGF acts as a molecular on-switch for MET, but it wasn’t clear until now whether METex14 needed to be stimulated by this molecular accelerator pedal to function, or if it was always active due to its missing brake pedal.
Next, the team studied the effect of METex14 on tumours in mice. Tumours with the faulty METex14 grew more quickly than those with normal MET, particularly in mice genetically engineered to express the human version of HGF needed to activate it. What’s more, treatment with MET-inhibitors reduced tumour growth in the mice, showing that this therapy works in this context.
Uncovering clues to why MET-inhibitors succeed or fail in treating cancer
Finally, the team examined lung tumour samples from 18 people with the METex14 mutation and found that HGF was present in two-thirds of tumours. “This raises an interesting question: if HGF is only found in some tumours, does its presence or absence partially explain why MET inhibitors work in some patients and not others?” Dr Kermorgant asks. “If so, it could be important to base treatment decisions not only on whether a person has this METex14 mutation but also on the level of HGF in the tumour.”
The team plans to test this theory further by examining a larger number of people with cancer, looking at whether the levels of HGF in a person’s tumour are linked to how effective MET inhibitors are for that individual. They also intend to create cell and animal models that pair METex14 with other genetic mutations common in cancer to examine the combined effect.
Ultimately, this work is helping to build a rulebook for personalising treatment for patients. This will equip doctors to select the optimum treatment approach for the unique properties of each tumour, giving people the best chance of living longer, healthier lives free from cancer.
Dr Kermorgant’s work on this study was funded by the Medical Research Council (MRC), OCTIMET Oncology, Rosetrees Trust, the Biotechnology and Biological Sciences Research Council (BBSRC) and Barts Charity. For a full list of funders, please see the research paper.
Category: General News, Publications

Ian Hart 20/06/2023
Well done Stephanie! A really nice contribution and some lovely results; look forward to seeing follow-up studies. Many congratulations to the whole team.
Stephanie 24/06/2023
Thanks so much Ian for your support!