New tool predicts risk of skin cancer spread more accurately than human inspection
A nationwide, cross-disciplinary effort led by researchers at Queen Mary University of London has developed a tool that predicts whether a type of skin cancer will spread. This could assist doctors in better treating individuals most at risk of aggressive disease. Teams in London, Glasgow, Southampton and Gloucestershire amassed samples from more than 200 people with skin cancer. Together, they harnessed machine learning to create a model that uses the activity of 20 genes to predict the risk of cancer spreading with more than 85% accuracy – surpassing current, unreliable human assessments of tumour diagnostic tissue. Ultimately, this tool could guide how we harness new immunotherapies for this type of skin cancer and help more people to survive their disease for longer.
Each year, around 50,000 people in the UK are diagnosed with Cutaneous Squamous Cell Carcinoma (cSCC), a type of skin cancer. The burden of the disease is growing, with diagnosis rising by about 5% annually as our population ages. While we can treat most cases of cSCC successfully, around 1 in 20 cases will metastasise, spreading to other areas of the body. Once the disease has spread, treatment becomes far more challenging and survival dramatically declines, with less than 30% of people surviving metastatic disease over five years.
If we could predict whose cancer is likely to spread, we could more closely focus on and monitor these individuals, giving them the additional treatment they need to control their disease. For example, a new immunotherapy called Cemiplimab was approved for treating advanced cSCC in the EU in 2019 that is showing positive results.
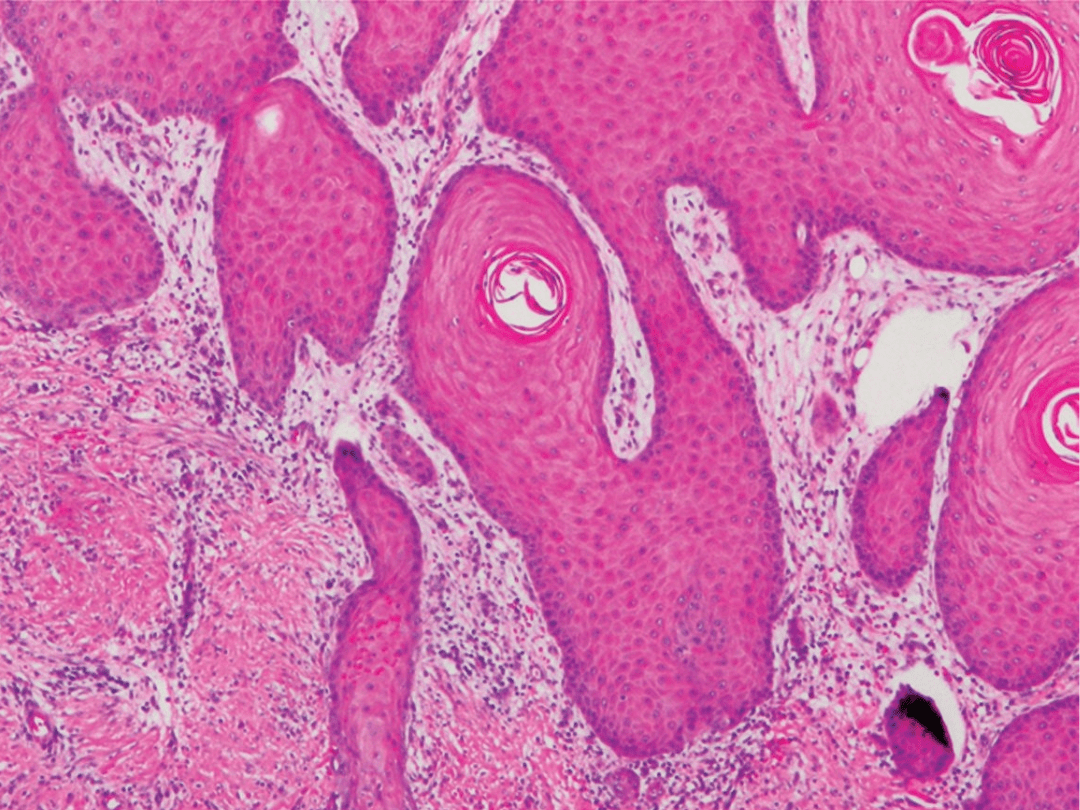
Currently, pathologists examine images of each tumour to estimate the risk that it will spread, but this can be a highly subjective process that yields unreliable predictions. The question remains: how can we make better predictions and focus treatment on the right patients?
“Without all of us working together, it wouldn’t have been possible.”
Dr Jun Wang, group leader at the Barts Cancer Institute, is the lead and corresponding author of a new study published in the Journal of the American Academy of Dermatology that reveals a promising new approach.
The study unites cSCC researchers from London, Glasgow, Southampton and Gloucestershire. It emerged from the UK cSCC clinical network led by Professor Irene M. Leigh CBE – a prominent leader in skin cancer biology research associated with Queen Mary University of London and a senior author on the new study.
“This network enabled us to pull together a large cohort of clinical samples from people with cSCC for the first time. It was a national effort,” comments Dr Wang. “It was also a multidisciplinary collaboration bringing together clinicians, bioinformaticians, biologists and pathologists. Without all of us working together, it wouldn’t have been possible.”
Identifying the tell-tale signs of cancer spread
The team collected tumour tissue samples from 237 people with cSCC across the four centres. In each sample, they used an approach called transcriptomics to look at how the cells’ more than 20,000 genes were activated or deactivated. This approach catalogues all of the RNA molecules (transcripts) that a cell creates to turn the instructions in its DNA into action – for example, producing proteins or regulating the cell’s function.
Dr Wang and team analysed this complex soup of transcripts in tumours that spread vs those that did not spread. They used state-of-the-art machine learning to reveal tell-tale differences between the two groups. This revealed a set of 20 genes whose activity levels together formed a signature indicating whether a tumour is likely to spread. This signature could predict metastasis with 86% accuracy, giving a more accurate and unbiased result compared with current, more subjective, assessment methods.
Professor Leigh comments: “We created a tissue bank that is unparalleled internationally, giving rise to these exciting findings of a transcriptomic signature associated with metastasis. The ability to identify high risk patients by a transcriptomic signature means that immunotherapy and other treatments can be targeted and clinical pathways refined to match potential outcomes.”
“We created a tissue bank that is unparalleled internationally.”
— Professor Irene M. Leigh
The next steps for the team will involve validating these results in a larger and more diverse patient cohort. Dr Wang is also eager to investigate some of the 20 genes highlighted in the new model to gain a better understanding of their role in cancer and whether they present promising new targets for therapies.
If validated through further testing, this model could provide an effective and unbiased means to reveal which patients with cSCC are at the highest risk of cancer spread. Ultimately, this would enable doctors to tailor their treatment and help more people with advanced cSCC to survive their disease for longer.
This work was made possible thanks to funding from Sanofi, Cancer Research UK, Barts Charity and the Academy of Medical Sciences.
Category: General News, Publications

No comments yet